Hepatitis B Vaccine HBAI20 Produced Enhanced Seroprotection For Non-Responders

A clinical-stage biotech company based in the Netherlands released positive results today from the Hepatitis B virus (HBV) vaccine, HBAI20, in Phase 2 “BE-Responder” trial.
The BE-Responder study is focused on improving the immunogenicity of current HBV vaccines by enhancing the adjuvant.
Approximately 5 percent of the healthy adult population responds inadequately to the commercial recombinant hepatitis B vaccines with an aluminum-based adjuvant.
This trial focused on so-called ‘non-responders’, who are persons who have been vaccinated with at least 1 complete vaccination course (3 injections of a licensed hepatitis B vaccine) without achieving a protective immune response.
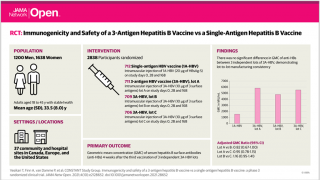
In this study of 133 hepatitis B vaccine non-responders, the HBAI20 vaccine is able to elicit protective anti‐HBs titres in 92% of nonresponders, 1 month after the third vaccination, versus 79%
in the HBVaxPro10 group.
In a previous study, also healthy adults who had never been vaccinated participated and were split into experimental and standard vaccine sections.
In the vaccine-naive group, all the recipients seroconverted by 7 months, but there was a major difference after the second of 3 shots where the experimental vaccine gave a 92% seroconversion rate compared to 58% of the standard vaccine.
This could potentially help the people who are hard to track and monitor and fail to get their third shots.
The adjuvant for the HBAI20 vaccine consisted of 20 µg of depot-formulated Interleukin-2 aggregates.
According to the World Health Organization (WHO), 257 million people suffer from chronic hepatitis B infection.
And treating hepatitis B can be expensive.
The WHO estimates that 15‐40 percent of patients with hepatitis B will develop cirrhosis, liver failure or hepatocellular carcinoma, thus creating a significant burden to healthcare systems due to high morbidity, mortality and costs of treatment.
Hepatitis B vaccines have been available since 1982. HBV vaccines are reported to be 95 percent effective in preventing infection and the development of chronic disease and liver cancer due to hepatitis B says the WHO.
In the USA, there are 3 single-antigen vaccines and 2 combination HBV vaccines available:
Single-antigen hepatitis B vaccines:
Combination vaccines:
To schedule a vaccination appointment at a local healthcare provider, please click here.
Vaccine financial support programs can be found at Vaccine Discounts.
CyTuVax has developed a platform vaccination technology, based on depot-formulated cytokines as an adjuvant.
These adjuvants can be applied in prophylactic vaccines against viral and bacterial antigens, while this vaccination technology is also developed for therapeutic indications in oncology.
After the success of this phase 2 clinical trial, CyTuVax is seeking partners to extend the use of its adjuvant technology platform to other vaccines.
For more information contact René Vleugels, managing director:
- T: +31 433881424, M: +31 627041717
- E: vleugels@cytuvax.com
Our Trust Standards: Medical Advisory Committee