$41.3 Million Supports Increased Access to the First Chikungunya Vaccine

As the most challenging disease to spell and pronounce continues its global expansion, access to the only approved chikungunya virus vaccine is becoming broader.
The Coalition for Epidemic Preparedness Innovations (CEPI) has expanded its support to provide expanded access to the world’s first chikungunya vaccine, IXCHIQ®.
IXCHIQ (VLA1553) is a monovalent, single-dose, live-attenuated chikungunya vaccine that the U.S. Food and Drug Administration approved in November 2023.
CEPI confirmed today that it will provide Valneva SE with up to $41.3 million in additional funding over the next five years, with support from the European Union’s (EU) Horizon Europe program.
The project aims to generate additional data to potentially support extended IXCHIQ® labels in chikungunya-endemic countries and vulnerable populations at risk of infection with this debilitating mosquito-borne disease.
This grant will also support post-marketing (phase 4) clinical trials and potential vaccine label extensions in children, adolescents, and pregnant women.
An earlier agreement awarded Valneva $24.6 million in CEPI-EU funding.
Thomas Lingelbach, Chief Executive Officer of Valneva, commented in a press release on July 22, 2024, “We are extremely pleased to strengthen our partnership with CEPI. Chikungunya infection is a major unmet medical need, and we believe that our single-dose vaccine is uniquely positioned to help protect people living in areas where chikungunya occurs and for travelers to these regions."
"With climate change, more areas across the world are becoming habitable for the mosquito vectors that transmit the virus, thereby increasing the size of the human population at risk of infection.”
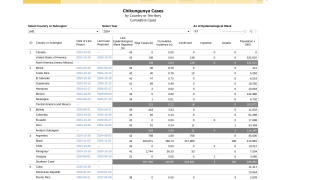
The World Health Organization has identified over 100 countries reporting chikungun cases in 2024, primarily in the Region of the Americas. As of July 22, 2024, the Pan American Health Organization had reported over 354,000 CHIKV cases, primarily in Brazil.
Even small island countries in the Indian Ocean have reported chikungunya outbreaks this year, such as in the Malé and Hulhumalé regions of Maldives.
The U.S. CDC recommends vaccination for chikungunya for adults (≥ 18 yrs) traveling to a destination with a current outbreak. IXCHIQ vaccination services are available in the U.S. at select pharmacies and travel clinics.
Our Trust Standards: Medical Advisory Committee