Africa's Mpox Plan Updated
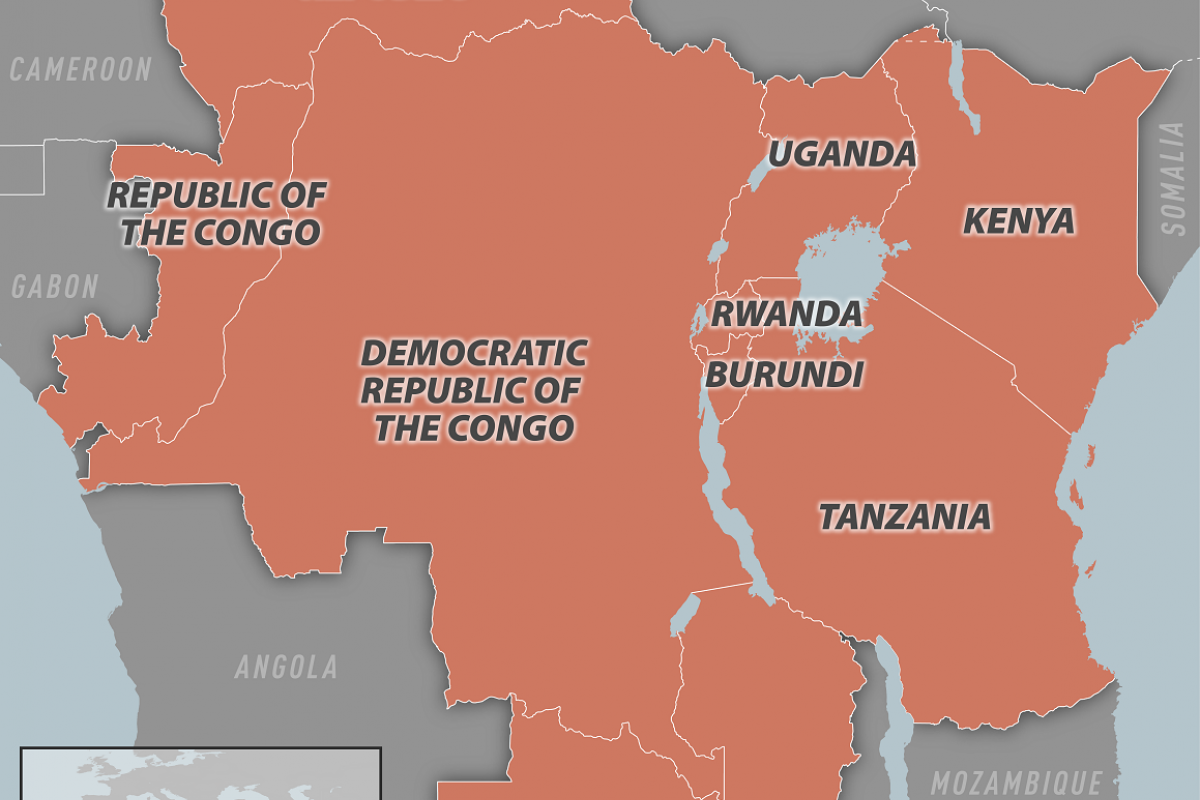
Since the declaration of the emergency, both regional and global support has increased, particularly for the Democratic Republic of the Congo (DRC), the epicentre of the ongoing mpox outbreak. Despite progress, significant challenges remain.
In the first two months of 2025, 60 countries reported mpox, with the majority of cases and deaths reported from the African continent.
The Africa CDC and WHO Joint Continental Mpox Plan has guided efforts to reduce this outbreak, focusing on ten key pillars: coordination, risk communication and community engagement, disease surveillance, laboratory capacity, clinical management, infection prevention and control, vaccination, research, logistics, and maintaining essential health services.
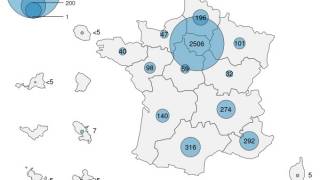
As of April 20, 2025, mpox vaccination efforts are underway, with more than 650,000 doses administered in six countries, 90% of which have been administered in the DRC.
Overall, over a million doses have been delivered to 10 countries, with efforts ongoing to secure additional vaccine supplies.
The leading mpox vaccine, JYNNEOS® (MVA-BN®, IMVAMUNE®), is produced by Bavarian Nordic A/S.
Along with the Continental Response Plan for Africa, the WHO has updated the global strategic plan to curb – and, where feasible, to stop – human-to-human transmission of mpox. The joint Continental Response Plan is aligned with the global strategy.
To alert international visitors of the ongoing mpox risk in Africa, the U.S. CDC issued a Level 2 - Practice Enhanced Precautions, Travel Health Advisory on April 1, 2025.
The CDC says people usually get mpox through intimate or close contact, including sex, with an infected person.
Mpox vaccination is recommended for people who anticipate sexual activities during travel to countries with ongoing person-to-person transmission of clade I mpox. In the U.S., the JYNNEOS vaccine is commercially available at clinics and pharmacies as of 2025.
Our Trust Standards: Medical Advisory Committee