Mpox Therapy Lacks Efficacy
Throughout the last few years, various mpox therapeutics have been made available to treat infected individuals. While clinical study results were pending, various health agencies, including the U.S. Food and Drug Administration (FDA), approved their use.
For example, Tecovirimat's efficacy for treating smallpox was established based on data from the FDA Animal Rule and from a study involving 359 healthy adults.
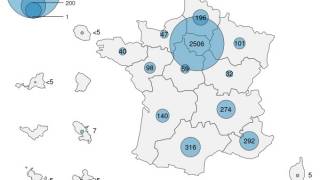
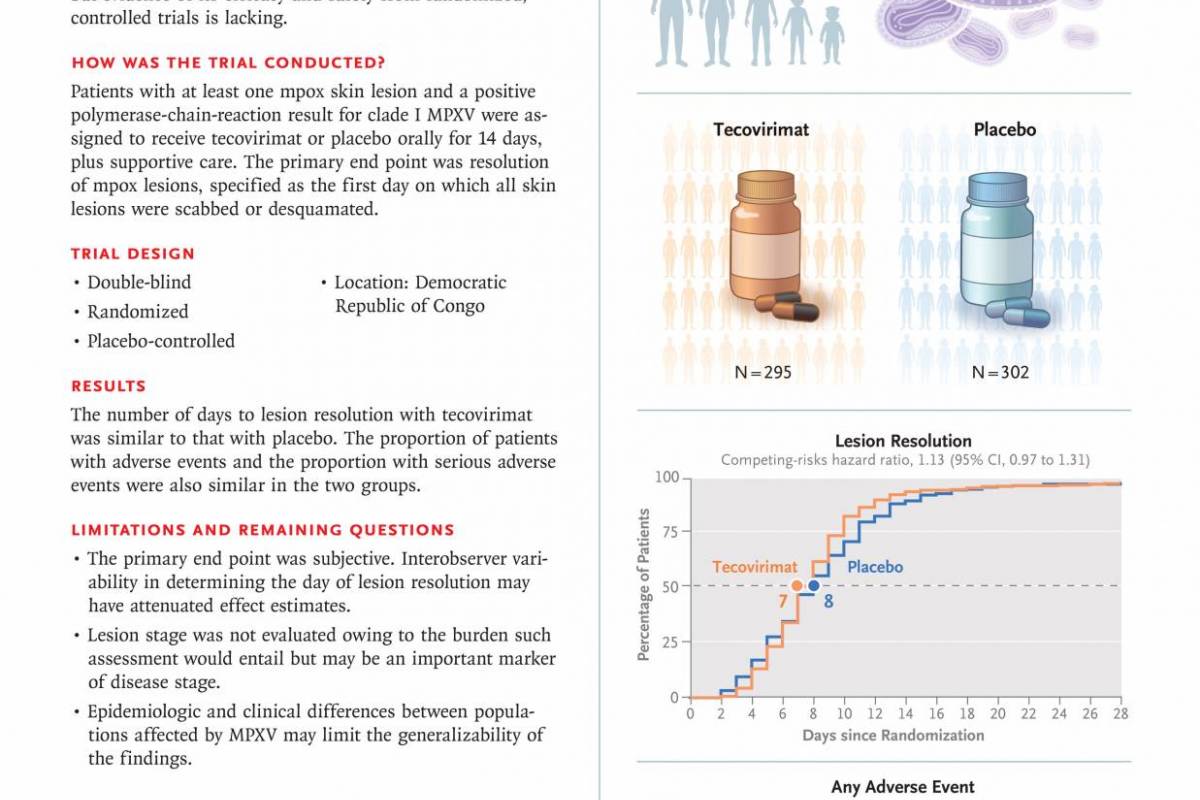
To confirm the efficacy of SIGA Technologies, Inc.'s TPOXX® (tecovirimat) for mpox patients, researchers conducted a double-blind, randomized, placebo-controlled trial funded by the National Institute of Allergy and Infectious Diseases and other organizations, using tecovirimat, in infected patients in the Democratic Republic of Congo (DRC).
Published by the New England Journal of Medicine on April 16, 2025, these researchers determined that tecovirimat did not reduce the number of days to lesion resolution in patients in the DRC with mpox caused by clade I MPXV.
This lack of real-world efficacy had been confirmed in previous mpox studies.
However, Mpox vaccinations with JYNNEOS® (MVA-BN®) have been shown to be effective in preventing clade I MPXV disease.
Our Trust Standards: Medical Advisory Committee