Shigellosis Vaccine Candidate Tested on Toddlers

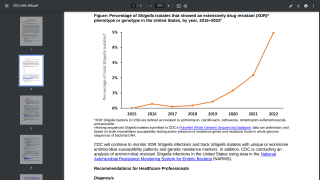
Shigellosis is the second leading cause of fatal diarrheal disease worldwide, strongly contributing to pediatric morbidity and mortality, without a U.S. FDA-approved vaccine available.
According to public health leaders and the Gates Foundation, developing an effective vaccine to prevent this deadly disease is essential in many areas worldwide.
To address this need, Valneva SE and LimmaTech Biologics AG today announced that the first participant has been vaccinated in a Phase 2 infant safety and immunogenicity study of Shigella4V2 (S4V2), the world's most clinically advanced tetravalent bioconjugate vaccine candidate against shigellosis.
Dr. Juan Carlos, Chief Medical Officer of Valneva, commented in a press release on April 9, 2025, "Seeing so many infants and children dying from shigellosis is not acceptable if it can be prevented with a vaccine."
"As such, the development of Shigella vaccines has been identified as a priority by the World Health Organization and, in line with our mission of developing vaccines against infectious diseases with unmet medical needs, we are focused on delivering a preventative solution against this deadly disease."
In the Phase 2 study S4V02, the safety and immunogenicity of S4V2 will be tested in approximately 110 nine-month-old infants to identify the best dose to be tested in a Phase 3 trial.
Sponsored and conducted by LimmaTech, S4V02 is a randomized, controlled, and blinded study conducted at a single study site in Kenya. Participants will receive a two-dose vaccination with one of two different vaccine dose levels of S4V2 or a control vaccine. Safety will be evaluated throughout the trial for approximately six months following the last vaccination.
Results of the phase 2 study, which is supported by funding from the Gates Foundation, are expected in the second half of 2025.
In November 2024, Valneva and LimmaTech launched a Phase 2b controlled human infection model (CHIM) study of S4V2 in healthy Shigella-naïve adults. This CHIM study forms part of the companies' staggered and risk-mitigating development strategy for S4V2, as it should provide the first results on efficacy before potentially advancing to further CHIM and Phase 3 studies.
Our Trust Standards: Medical Advisory Committee