Mpox Travel Advisory Expanded; Vaccinations Recommended
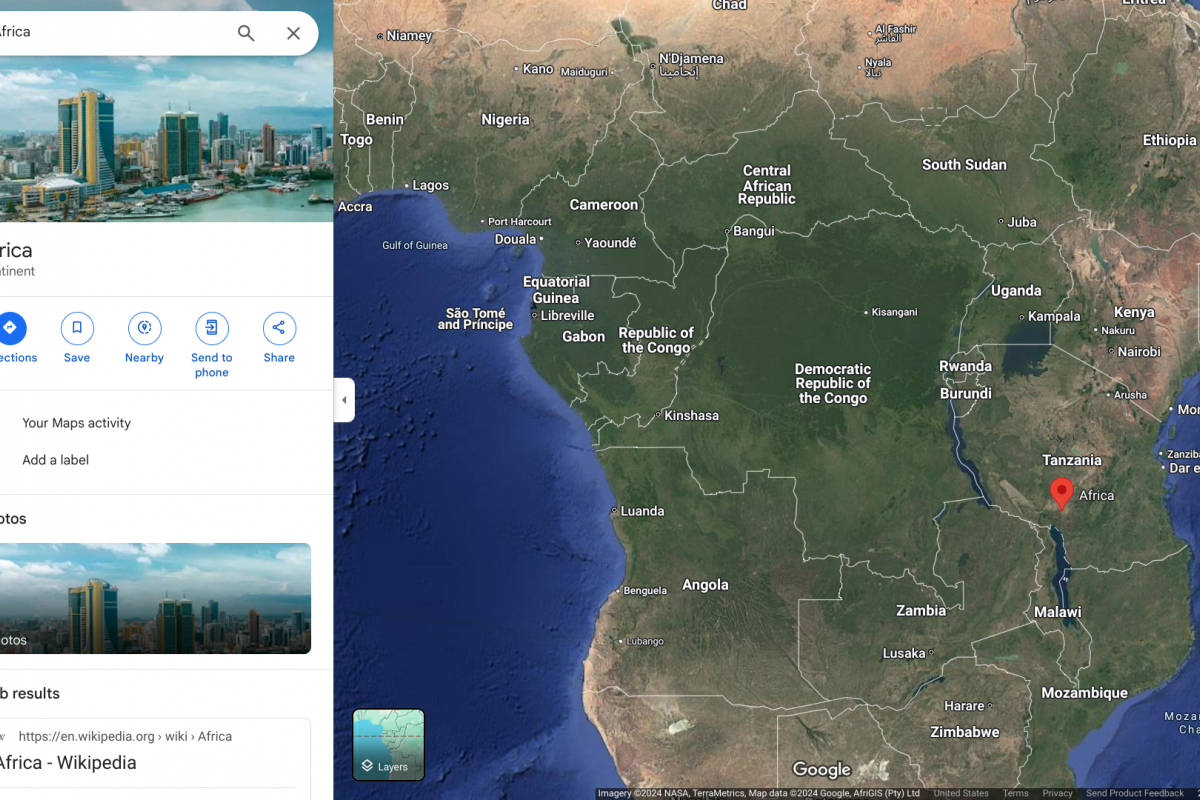
The U.S. Centers for Disease Control and Prevention (CDC) today announced active person-to-person transmission of mpox clade 1 in Burundi, Central African Republic, Democratic Republic of the Congo, Kenya, the Republic of the Congo, Rwanda, and Uganda.
As of December 30, 2024, the CDC's Level 2 Travel Health Advisory says person-to-person transmission has occurred through various means during this mpox outbreak. Historically, clade I have been associated with a higher percentage of people with mpox developing severe illness or dying, compared to clade II.
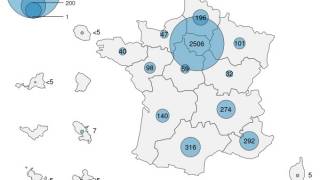
The mpox clade II outbreak began in the United States in late May 2022.
As of November 2024, there have been 2,455 confirmed clade II mpox cases this year, which has exceeded the 1,407 confirmed cases in 2023.
Furthermore, the CDC says mpox vaccination is recommended for certain people traveling to countries with ongoing person-to-person transmission of clade I mpox.
In the U.S., the JYNNEOS® (MVA-BN, IMVANEX®, IMVAMUNE®) Smallpox and Mpox Vaccine has been approved by the U.S. FDA since September 2019. Other mpox vaccines and vaccine candidates are actively seeking regulatory approvals in 2025.
Our Trust Standards: Medical Advisory Committee