MenB-4C Vaccine Dosing Schedule Updated

The U.S. CDC announced today that it supports the new Dosing Interval and Schedule for the Bexsero® MenB-4C Vaccine based on the Updated Recommendations of the Advisory Committee on Immunization Practices in October 2024.
On December 12, 2024, the CDC's MMWR (73(49);1124–1128) recommended MenB-4C as a 2-dose series with doses administered at intervals of 0 and 6 months for healthy adolescents and young adults aged 16–23 based on shared clinical decision-making and as a 3-dose series with doses administered at 0, 1–2, and 6 months for persons aged ≥10 years at increased risk.
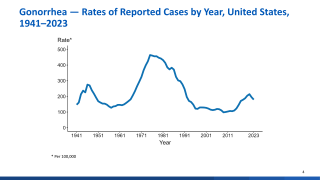
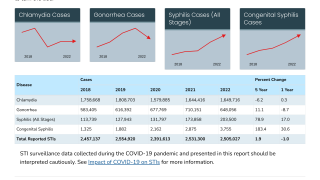
This news is very relevant for teenagers and college students as real-world evidence suggests that Bexsero provides cross-protection against gonorrhea, a sexually transmitted disease.
For example, the U.K.'s Joint Committee on Vaccination and Immunisation recommended a routine targeted vaccination program in 2023 using the 4CMenB to prevent gonorrhea.
Our Trust Standards: Medical Advisory Committee