Rabies Vaccine Coring Process Needs Close Attention

With more than 150 countries and territories reporting rabies cases, accessing one of the four authorized vaccines is essential to reducing this viral disease, says the World Health Organization.
Bavarian Nordic recently announced it is in agreement with the U.K.'s Medicines and Healthcare products Regulatory Agency (MHRA) regarding reports of rubber particles after reconstitution of the Rabipur rabies vaccine.
Bavarian Nordic has recently received an unexpected number of product quality complaints about visual particles in the vaccine solution.
Recommendations, issued on August 14, 2024, have been provided to minimize health risks. The analysis revealed that these particles consisted of rubber transferred from the rubber stopper of the vaccine vials during (coring) reconstitution.
The MHRA's letter to healthcare providers recommends that the reconstituted Rabipur vaccine be carefully inspected visually and not administered if visible particles are present. Suspected adverse drug reactions can be reported to the MHRA through the Yellow Card scheme.
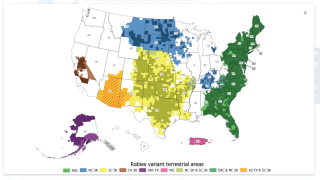
While most of the world traces rabies infections in people to bites from an infected dog, in the United States, rabid bats are the leading source of infection.
Our Trust Standards: Medical Advisory Committee